Health Care Regulatory Explorer
Medical Device Regulatory Support - 24x7
From concept to launch - Regtik delivers correct, fast regulatory guidance on medical device qualification and classification.
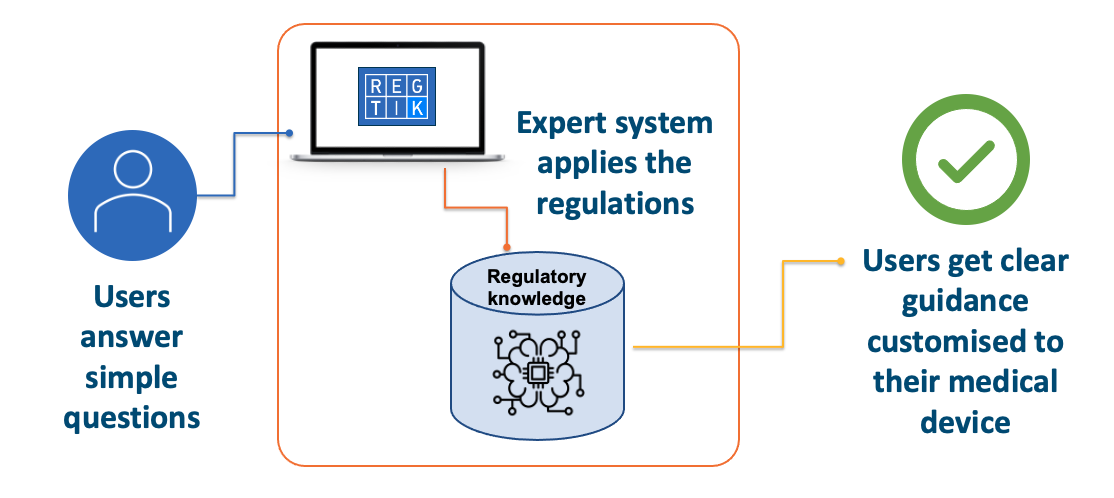
Our easy-to-use platform lets you explore the regulatory landscape and find out how design decisions can change your regulatory options. Find out in minutes:
- Qualification – whether your product counts as a regulated medical device
- Classification – the risk class of your medical device (e.g. Class I, IIa, IIb, III) under the regulations
- Regulatory pathways – the steps you need to take to go to market in the UK, EU, USA and Australia
We know that early stage concepts can change – you can run product changes through the system as often as you like at no extra cost.
Use our AI-powered guidance to build a regulatory strategy for licensing and launch across key global markets: the UK, EU, USA and Australia.
Sign up below!
